Cannabis testing laboratories around the country are expanding quickly, taking on new clients and growing their business incrementally. Many of these labs are receiving a large number of test requests from growers for potency testing, terpene profiling, pesticide screening, residual solvent screening, heavy metal testing, microbial analysis and even genetic testing. To keep pace with the number of test requests received, efficient data, sample and test management is imperative.
Considering the magnitude of cannabis testing, data management using spreadsheets is a serious impediment to quality assurance. Data being recorded in spreadsheets is error-prone and difficult to manage. Furthermore, using spreadsheets does not allow labs to adhere to regulatory guidelines that demand strict accounting for every gram of the sample, right from reception, consumption for testing, to disposal.
To overcome such data management challenges and improve the operational efficiency of cannabis testing laboratories, a Laboratory Information Management System (LIMS) plays a significant role. LIMS are much more capable than spreadsheets and paper-based tools for managing analytical and operational activities. LIMS enhances the productivity and quality by eliminating the manual data entry. With its built-in audit trail capability, LIMS helps labs adhere to regulatory standards.
LIMS can provide companies with a method to manage samples, records and test results, and ensures regulatory compliance by increasing traceability. LIMS can also be integrated with other lab instrumentation and enterprise systems, enabling easier transmission of information across the lab and the organization, reducing manual efforts and improving decision-making.
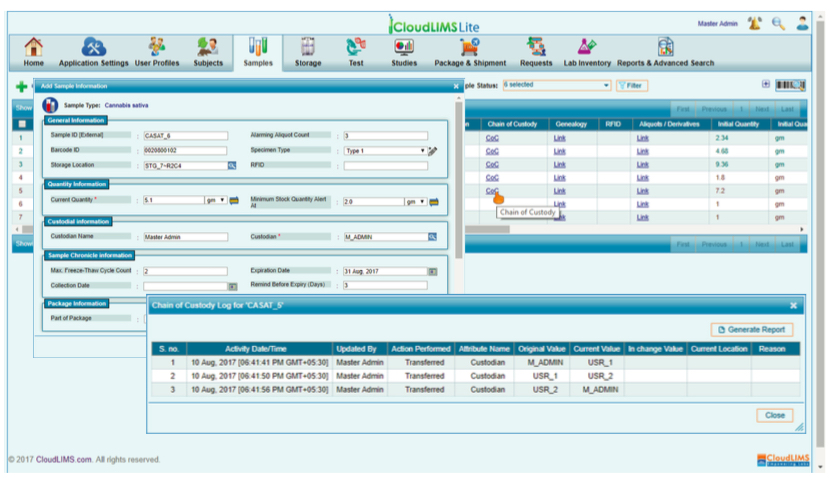
Multiple resources are also available to assist labs in preparing for quality assurance and accreditation, LIMS being one of them. LIMS can help cannabis labs with instrument integration, and automate reporting to help improve efficiencies and reduce errors. LIMS, such as CloudLIMS Lite, a cloud-based LIMS, automates cannabis-testing workflows right from sample collection, data recording, managing test chain of custody, sample weight accounting to report generation. With data security and audit trails, a LIMS provides traceable documentary evidence required to achieve ISO 17025 accreditation for highly regulated labs. Above all, cloud-enabled systems are often low in the total cost of acquisition, have maintenance outsourced, and are scalable to help meet the ever-changing business and regulatory compliance needs.
Cloud-based products are secure, easy to deploy and scalable. A cloud product is typically hosted on a server with a guaranteed uptime of 99.5%, allowing for a reliable system, accessible 24×7. Cloud-based LIMS have automatic data backup mechanism that allow for quick turnarounds in case of a server failure or in the eventuality of a natural disaster.
With LIMS in place, cannabis labs can manage sample and requisition-centric records, track sample quantity and location, integrate the test data, and provide online reports to clients. This in turn, reduces the turnaround time for testing and improves the operational efficiency. Besides, audit trail of each and every activity performed by the lab personnel is recorded in the system to ensure that the lab follows regulatory compliance.
Editor’s Note: This is a condensed version of a poster that was submitted and displayed at this year’s Cannabis Science Conference in Portland, Oregon. The authors of the original poster are Arun Apte, Stephen Goldman, Aditi Gade and Shonali Paul.
Opinions expressed the above syndicated article by Aaron Biros are for informational purposes only and not necessarily the opinion of Herban Medical Options.
For full article and information, visit: Source